Acquisition systems
Confocal
Confocal microscopy, most frequently confocal laser scanning microscopy (CLSM) or laser confocal scanning microscopy (LCSM), is an optical imaging technique for increasing optical resolution and contrast of a micrograph by means of using a spatial pinhole to block out-of-focus light in image formation.[1] Capturing multiple two-dimensional images at different depths in a sample enables the reconstruction of three-dimensional structures (a process known as optical sectioning) within an object.
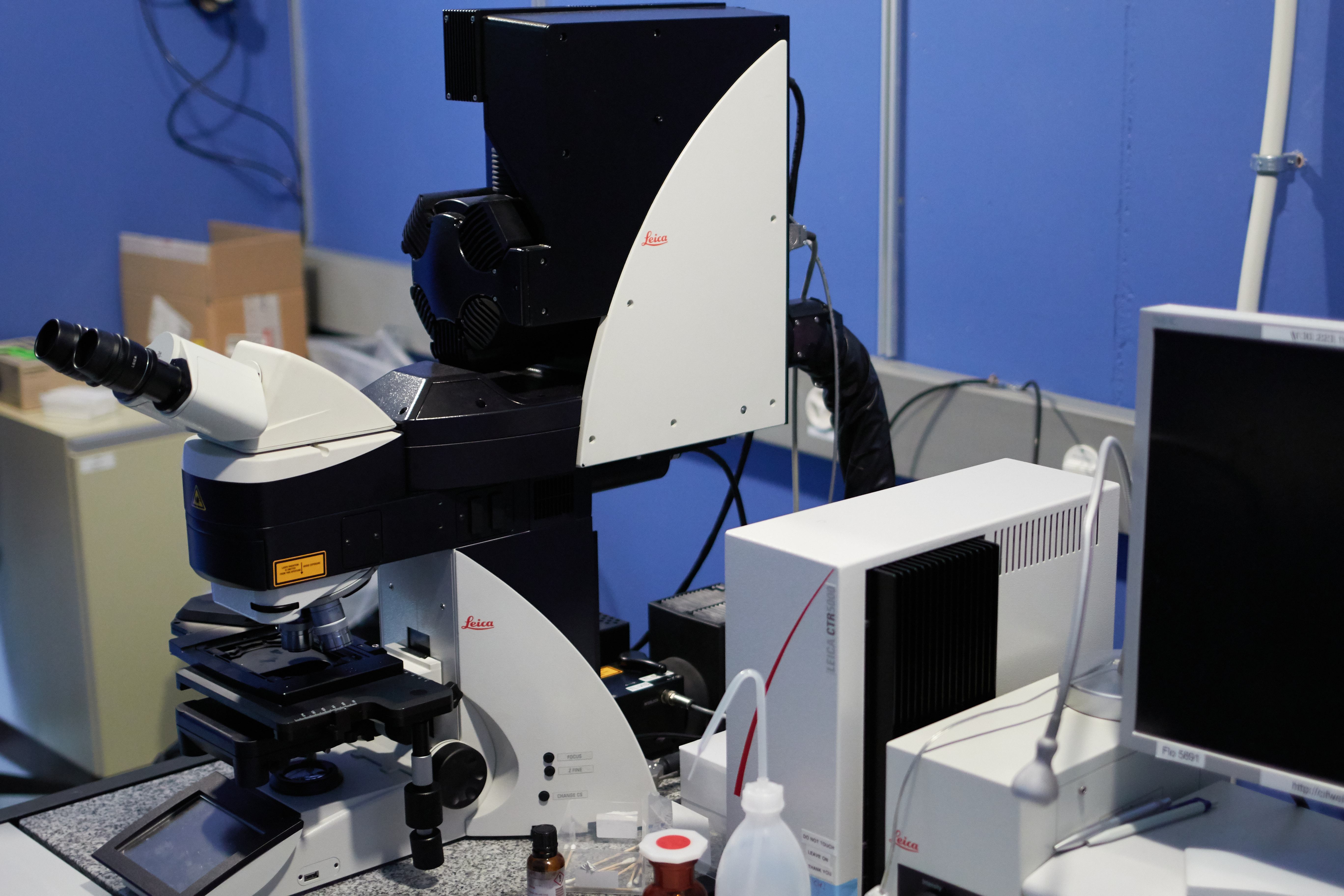
Zeiss LSM 800
| Stage |
|
|
Illumination |
|
|
Lasers |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Zeiss LSM 880 with Airyscan
| Stage |
|
|
Illumination |
|
|
Lasers |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Leica SP5
| Stage |
|
|
Illumination |
|
|
Lasers |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Zeiss LSM 800 P2 Lab [SPECIAL AUTHORIZATION REQUIRED]
This particular setup being placed in a P2 Lab, a special authorization is required to access it. Contact the technical manager for further information
| Stage |
|
|
Illumination |
|
|
Lasers |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Slide Scanner
A slide scanner allows you to digitize full microscopy slides in an automated manner. This is a high throughput system able to process around a hundred of slides in one batch.
The NanoZoomer S60 slide-scanner can work as an intuitive microscope designed for slides or as a regular slide-scanner. The way a slide scanner works implies that designing a specific profile for each of your sample type is needed.
This profile is very dependent of the way you have prepared your slides. Since this processe is time-consuming, it is only useful if you have at least a dozen or more slides of the exact same type to acquire (1-2 hours with testing).
Here are some general guidelines before thinking of using the slide scanner for your experiments:
|
SCAN PROFILE |
|---|
|
If the samples vary in any one of these categories:
Fluorescence specific:
The profile will have to be updated and saved as a new variant or it will probably fail (not focused, over/underexposed, stitching failing). |
|
ACQUISITION TIME |
|
The acquisition time depends on:
Each of these parameters can greatly vary the acquisition time, which can range from a few minutes to an hour or more per slide. |
Hamamatsu NanoZoomer S60
Brochure available here for more information : https://www.hamamatsu.com/resources/pdf/sys/SBIS0043E_NanoZoomers.pdf
|
Illumination |
|
|
Filters |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Cameras |
Color camera
Black and White Camera
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Widefield | Fluorescence
The majority of fluorescence microscopes, especially those used in the life sciences, are of the epifluorescence design shown in the diagram. Light of the excitation wavelength illuminates the specimen through the objective lens. The fluorescence emitted by the specimen is focused to the detector by the same objective that is used for the excitation which for greater resolution will need objective lens with higher numerical aperture. Since most of the excitation light is transmitted through the specimen, only reflected excitatory light reaches the objective together with the emitted light and the epifluorescence method therefore gives a high signal-to-noise ratio. The dichroic beamsplitter acts as a wavelength specific filter, transmitting fluoresced light through to the eyepiece or detector, but reflecting any remaining excitation light back towards the source.
Zeiss Axiovision Upright
| Stage |
|
|
Illumination |
|
|
Filter Cubes |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Cameras |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Zeiss Axiovision Upright B
| Stand |
|
|
Illumination |
|
|
Filters |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Cameras |
Color camera
Black and White Camera
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Time-Lapse microscopy
Time-lapse microscopy is time-lapse photography applied to microscopy. Microscope image sequences are recorded and then viewed at a greater speed to give an accelerated view of the microscopic process. Before the introduction of the video tape recorder in the 1960s, time-lapse microscopy recordings were made on photographic film. During this period, time-lapse microscopy was referred to as microcinematography. With the increasing use of video recorders, the term time-lapse video microscopy was gradually adopted. Today, the term video is increasingly dropped, reflecting that a digital still camera is used to record the individual image frames, instead of a video recorder.
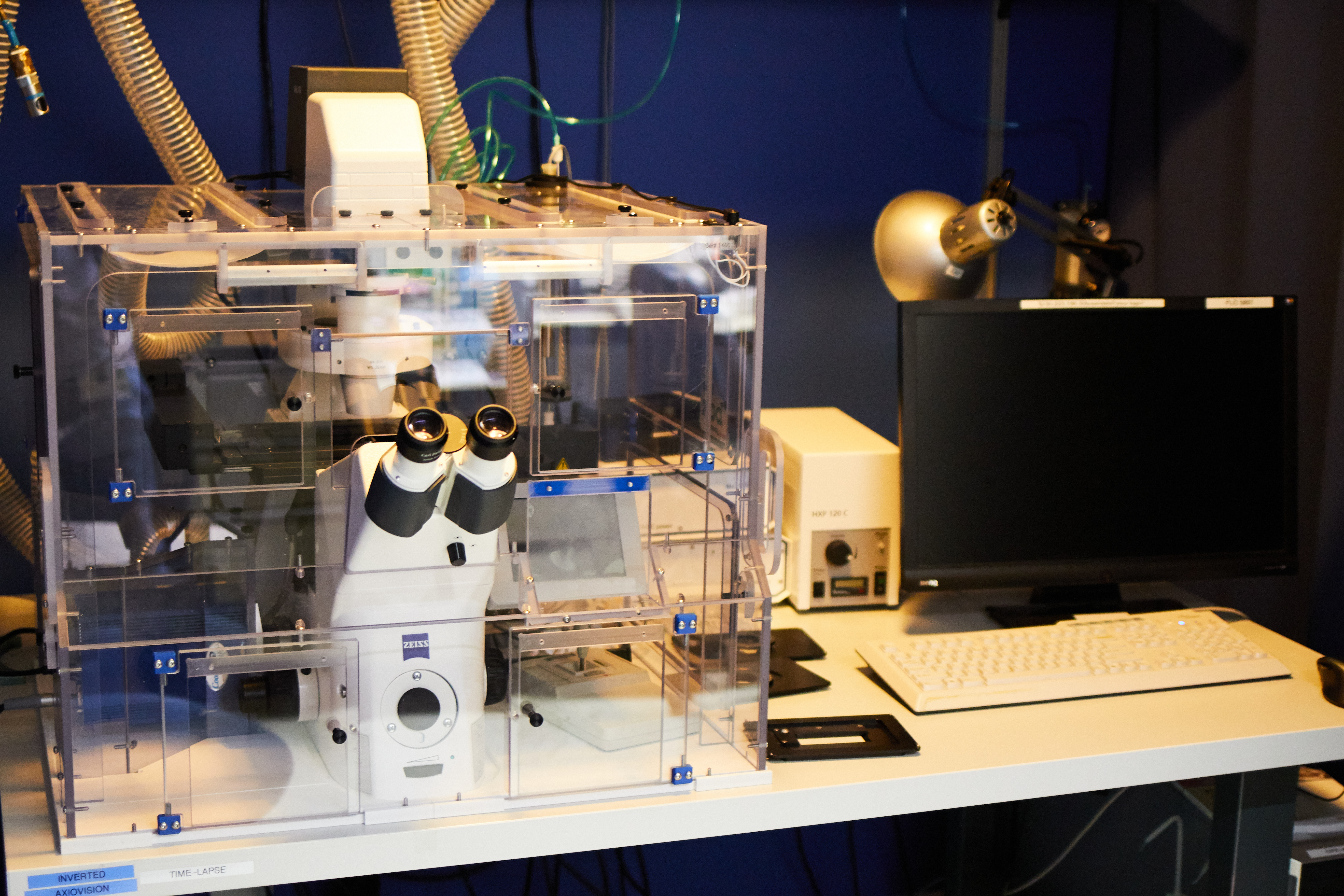
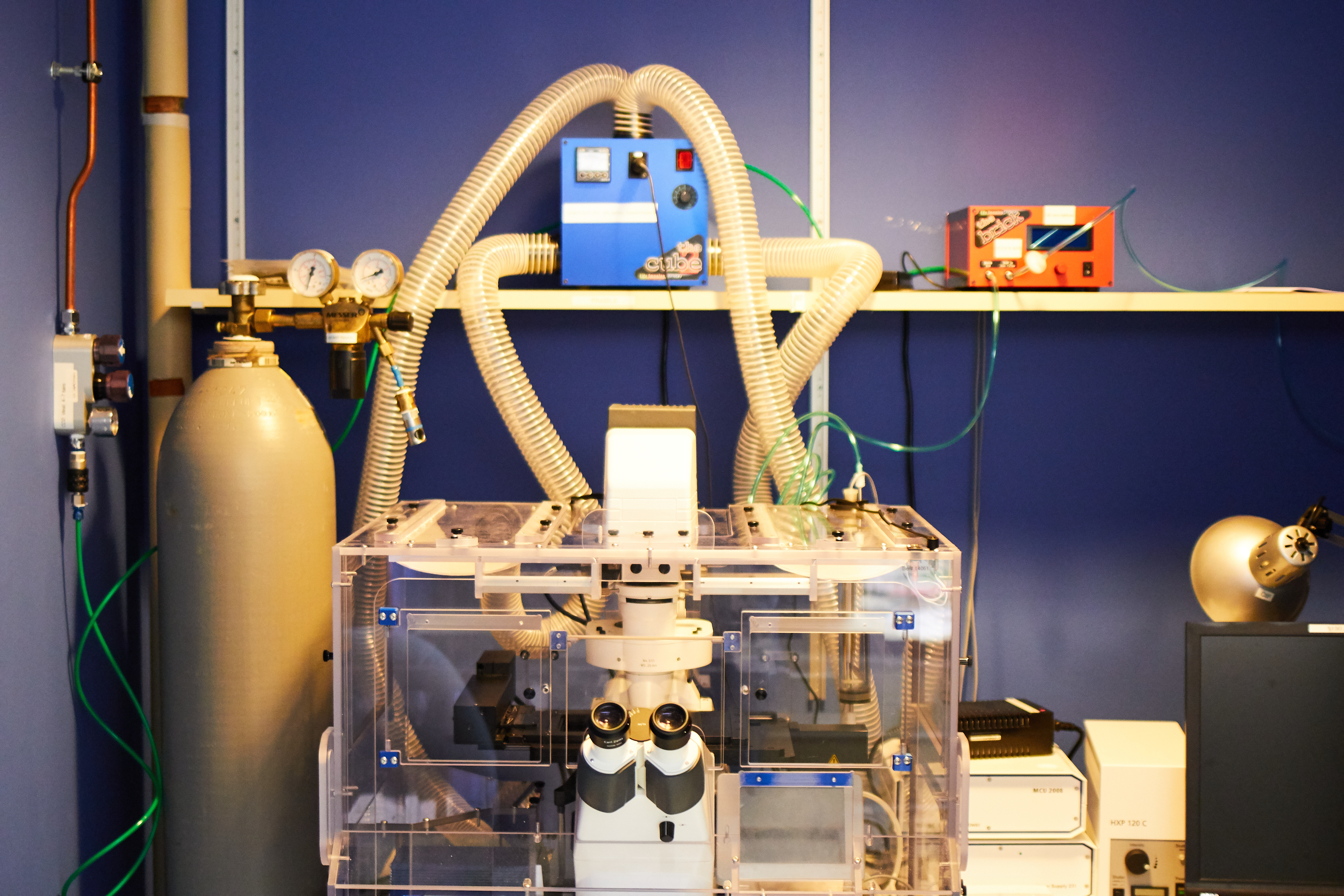
Zeiss Axiovision Inverted (Time-Lapse)
| Stage |
|
|
Illumination |
|
|
Filter Cubes |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Cameras |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Stereomicroscopy
The stereo, stereoscopic or dissecting microscope is an optical microscope variant designed for low magnification observation of a sample, typically using light reflected from the surface of an object rather than transmitted through it. The instrument uses two separate optical paths with two objectives and eyepieces to provide slightly different viewing angles to the left and right eyes. This arrangement produces a three-dimensional visualization of the sample being examined.[1] Stereomicroscopy overlaps macrophotography for recording and examining solid samples with complex surface topography, where a three-dimensional view is needed for analyzing the detail.
Leica M205-FA
| Stand |
|
|
Illumination |
|
|
Filters |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Cameras |
Color camera
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Light Sheet Microscopy
Light sheet fluorescence microscopy (LSFM) is a fluorescence microscopy technique with an intermediate-to-high[1]optical resolution, but good optical sectioning capabilities and high speed. In contrast to epifluorescence microscopy only a thin slice (usually a few hundred nanometers to a few micrometers) of the sample is illuminated perpendicularly to the direction of observation. For illumination, a laser light-sheet is used, i.e. a laser beam which is focused only in one direction (e.g. using a cylindrical lens). A second method uses a circular beam scanned in one direction to create the lightsheet. As only the actually observed section is illuminated, this method reduces the photodamage and stress induced on a living sample. Also the good optical sectioning capability reduces the background signal and thus creates images with higher contrast, comparable to confocal microscopy. Because LSFM scans samples by using a plane of light instead of a point (as in confocal microscopy), it can acquire images at speeds 100 to 1000 times faster than those offered by point-scanning methods.
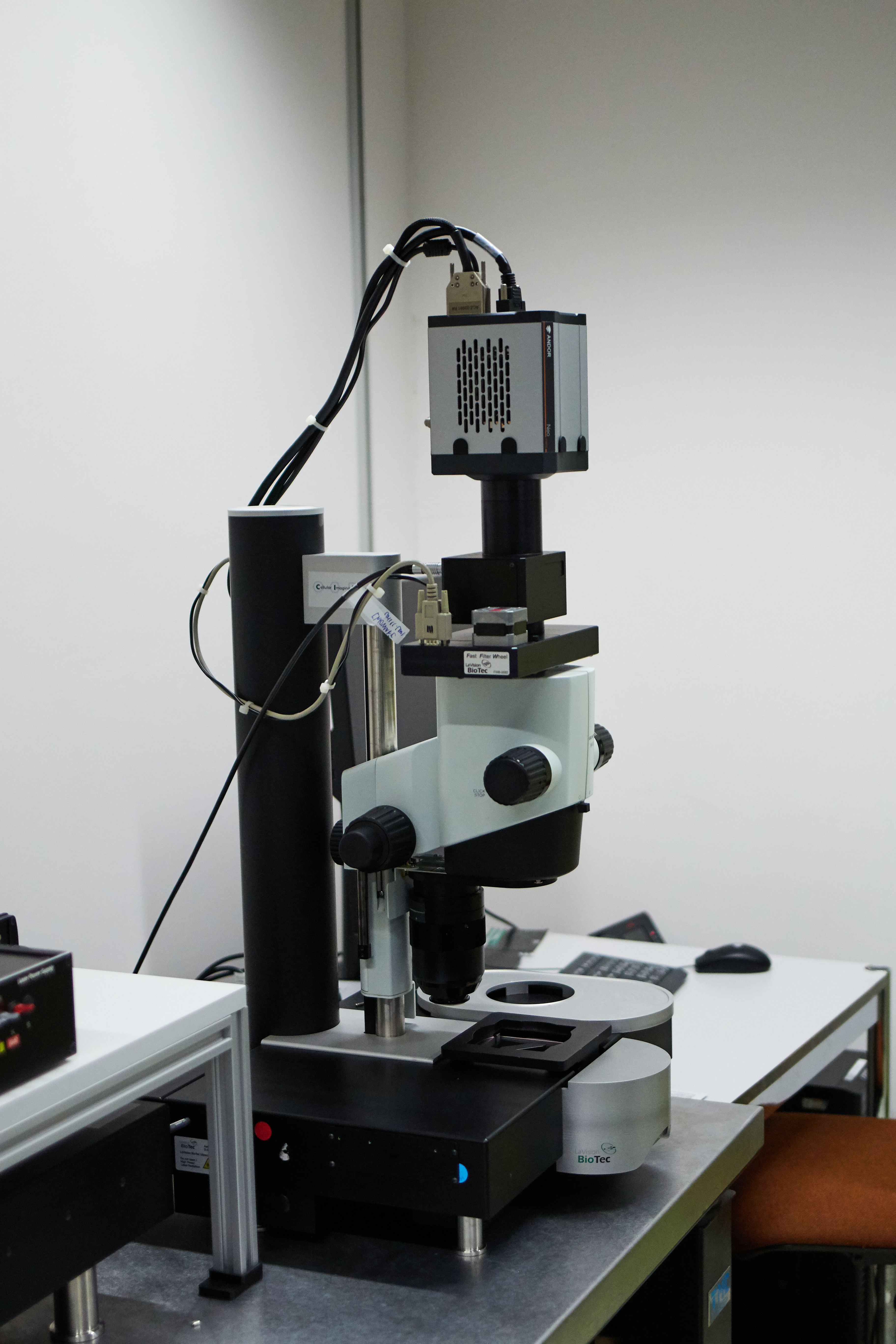
LaVision Biotec Ultramicroscope II
Laser-capture Microdissection
Laser-capture microdissection (LCM) is a method to procure subpopulations of tissue cells under direct microscopic visualization. LCM technology can harvest the cells of interest directly or can isolate specific cells by cutting away unwanted cells to give histologically pure enriched cell populations. A variety of downstream applications exist: DNA genotyping and loss-of-heterozygosity (LOH) analysis, RNA transcript profiling, cDNA library generation, proteomics discovery and signal-pathway profiling. The total time required to carry out this protocol is typically 1–1.5 h.[3]
Acturus LCM
| Stand |
|
|
Widefield Illumination |
|
| Filters |
|
|
Lasers |
Laser cutting
Optical tweezer
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Image Processing
[Under construction]