Acquisition systems
Confocal
Confocal microscopy, most frequently confocal laser scanning microscopy (CLSM) or laser confocal scanning microscopy (LCSM), is an optical imaging technique for increasing optical resolution and contrast of a micrograph by means of using a spatial pinhole to block out-of-focus light in image formation.[1] Capturing multiple two-dimensional images at different depths in a sample enables the reconstruction of three-dimensional structures (a process known as optical sectioning) within an object.
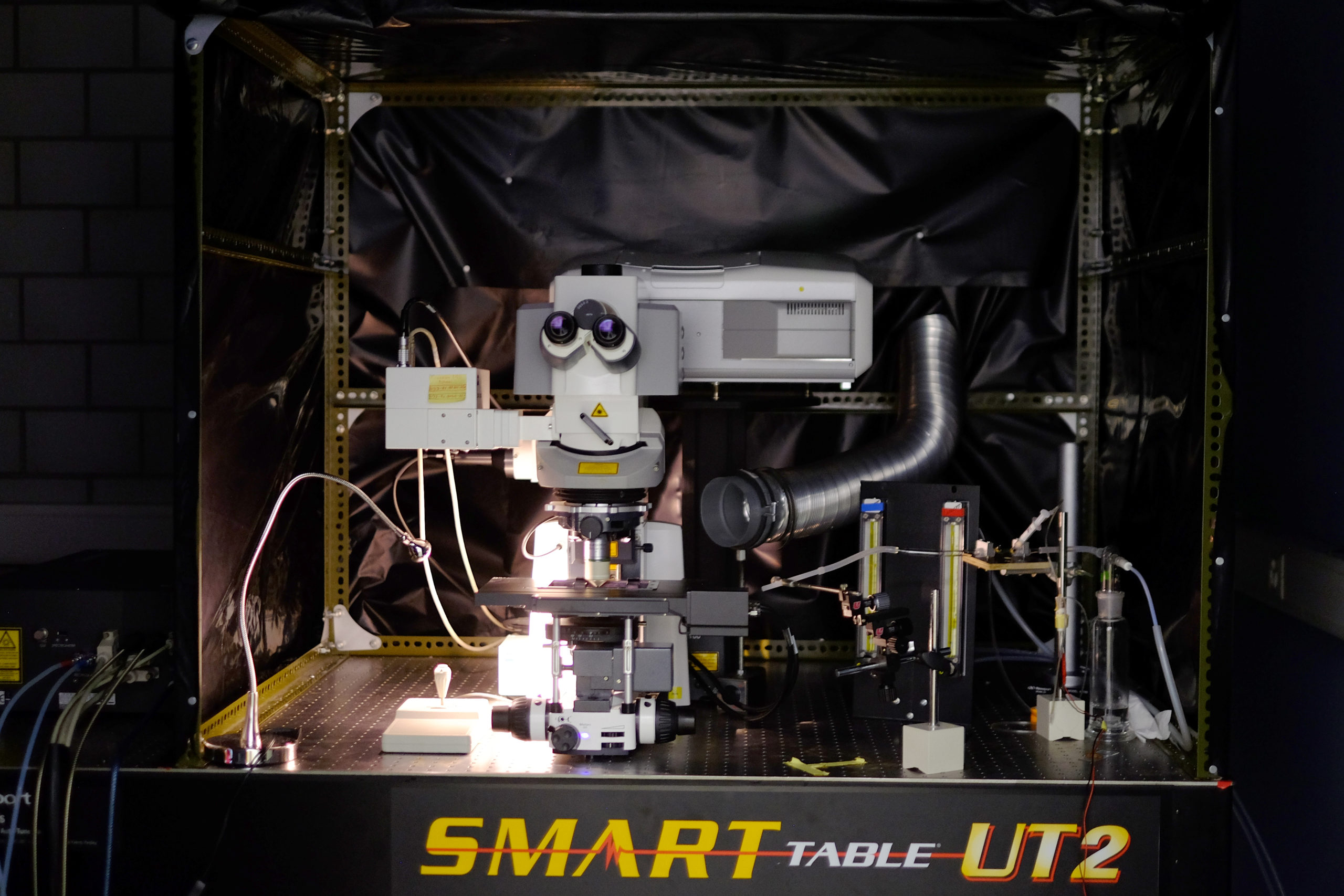
Zeiss LSM 880 Airyscan
| Stand |
|
|
Illumination |
|
|
Lasers |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Zeiss LSM 710
| Stand |
|
|
Illumination |
|
|
Lasers |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Leica Stellaris 5 WLL IR with Resonnant Scanner
| Stand |
|
|
Illumination |
|
|
Lasers |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Leica Stellaris 5 with Resonnant Scanner
| Stand |
|
|
Scanner |
|
|
Lasers |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Leica SP8X
| Stand |
|
|
Illumination |
|
|
Lasers |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Multi-Photon Confocal
Two-photon excitation microscopy is a fluorescence imaging technique that allows imaging of living tissue up to about one millimeter in depth. It differs from traditional fluorescence microscopy, in which the excitation wavelength is shorter than the emission wavelength, as the wavelengths of the two exciting photons are longer than the wavelength of the resulting emitted light. Two-photon excitation microscopy typically uses near-infrared excitation light which can also excite fluorescent dyes. However, for each excitation, two photons of infrared light are absorbed. Using infrared light minimizes scattering in the tissue. Due to the multiphoton absorption, the background signal is strongly suppressed. Both effects lead to an increased penetration depth for these microscopes. Two-photon excitation can be a superior alternative to confocal microscopy due to its deeper tissue penetration, efficient light detection, and reduced photobleaching.[1][2]
Zeiss LSM 710 NLO
| Stand |
|
|
Illumination |
|
|
Lasers |
1 Photon
2 Photon
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
Internal
External
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
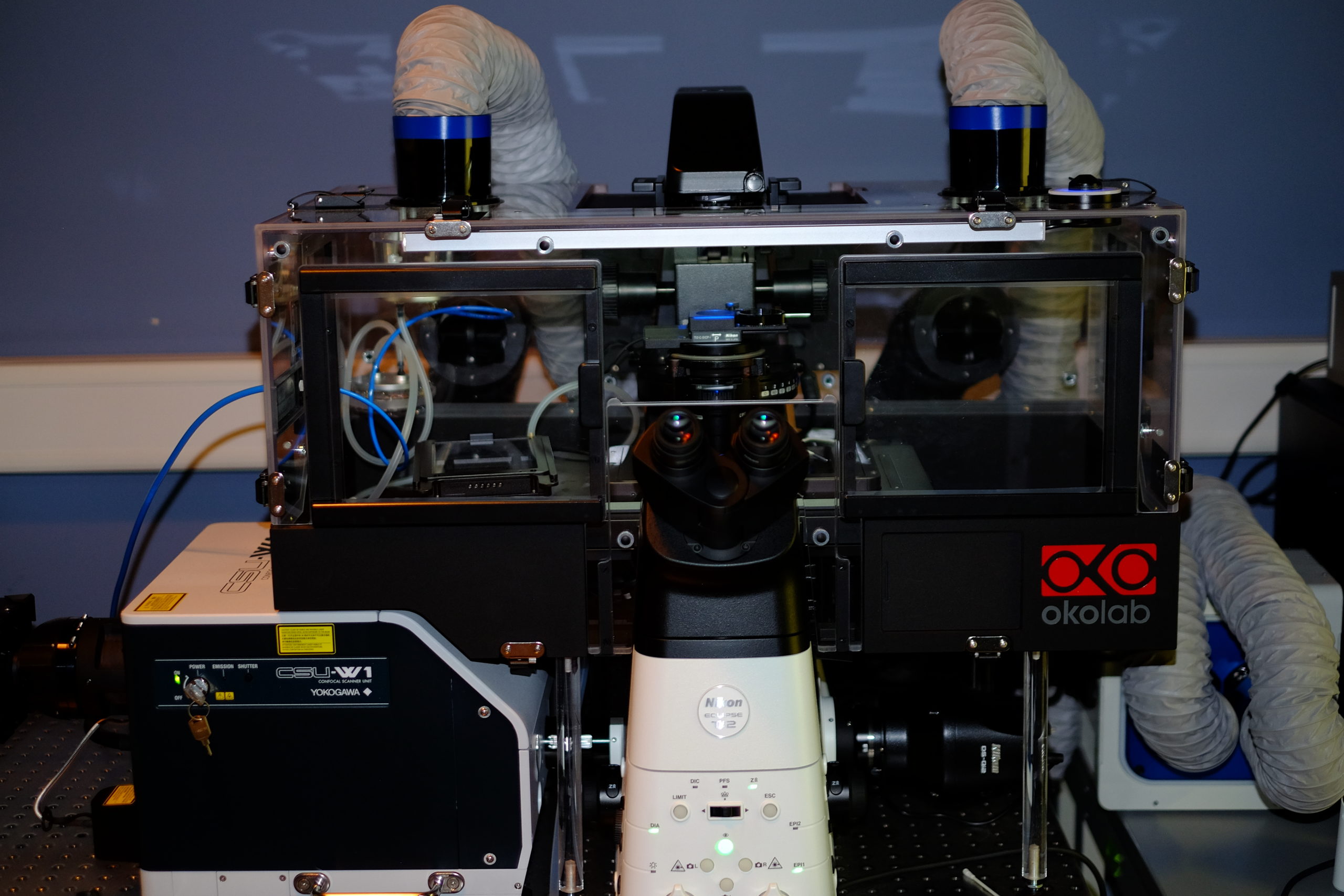
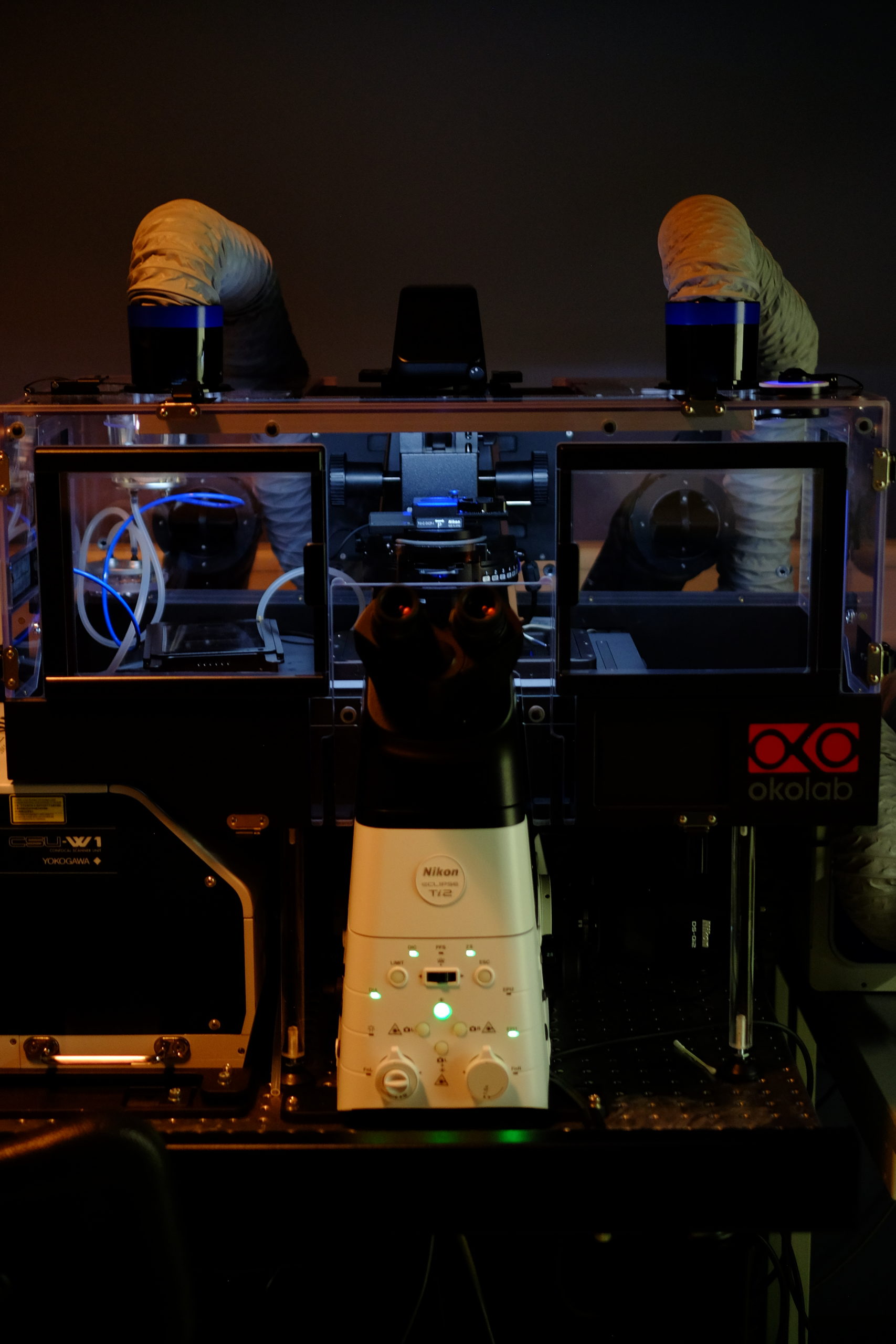
Spinning Disk | Time-Lapse
Spinning-disk (Nipkow disk) confocal microscopes use a series of moving pinholes on a disc to scan spots of light. Since a series of pinholes scans an area in parallel, each pinhole is allowed to hover over a specific area for a longer amount of time thereby reducing the excitation energy needed to illuminate a sample when compared to laser scanning microscopes. Decreased excitation energy reduces phototoxicity and photobleaching of a sample often making it the preferred system for imaging live cells or organisms.
Nikon Ti2 | Yokogawa CSU-W1
|
Widefield LED Illumination |
Lumencore Spectra X LED
|
|
Filter Cubes |
|
|
Spinning Disk laser excitation |
Omicron modified LightHUB+
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Cameras |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Laser-capture Microdissection
Laser-capture microdissection (LCM) is a method to procure subpopulations of tissue cells under direct microscopic visualization. LCM technology can harvest the cells of interest directly or can isolate specific cells by cutting away unwanted cells to give histologically pure enriched cell populations. A variety of downstream applications exist: DNA genotyping and loss-of-heterozygosity (LOH) analysis, RNA transcript profiling, cDNA library generation, proteomics discovery and signal-pathway profiling. The total time required to carry out this protocol is typically 1–1.5 h.[3]
MMI CellCut | Olympus IX71 inverted microsocope
|
Widefield Illumination |
|
|
Lasers |
Laser cutting
Optical tweezer
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Widefield | Fluorescence
The majority of fluorescence microscopes, especially those used in the life sciences, are of the epifluorescence design shown in the diagram. Light of the excitation wavelength illuminates the specimen through the objective lens. The fluorescence emitted by the specimen is focused to the detector by the same objective that is used for the excitation which for greater resolution will need objective lens with higher numerical aperture. Since most of the excitation light is transmitted through the specimen, only reflected excitatory light reaches the objective together with the emitted light and the epifluorescence method therefore gives a high signal-to-noise ratio. The dichroic beamsplitter acts as a wavelength specific filter, transmitting fluoresced light through to the eyepiece or detector, but reflecting any remaining excitation light back towards the source.
Nikon lab microscope
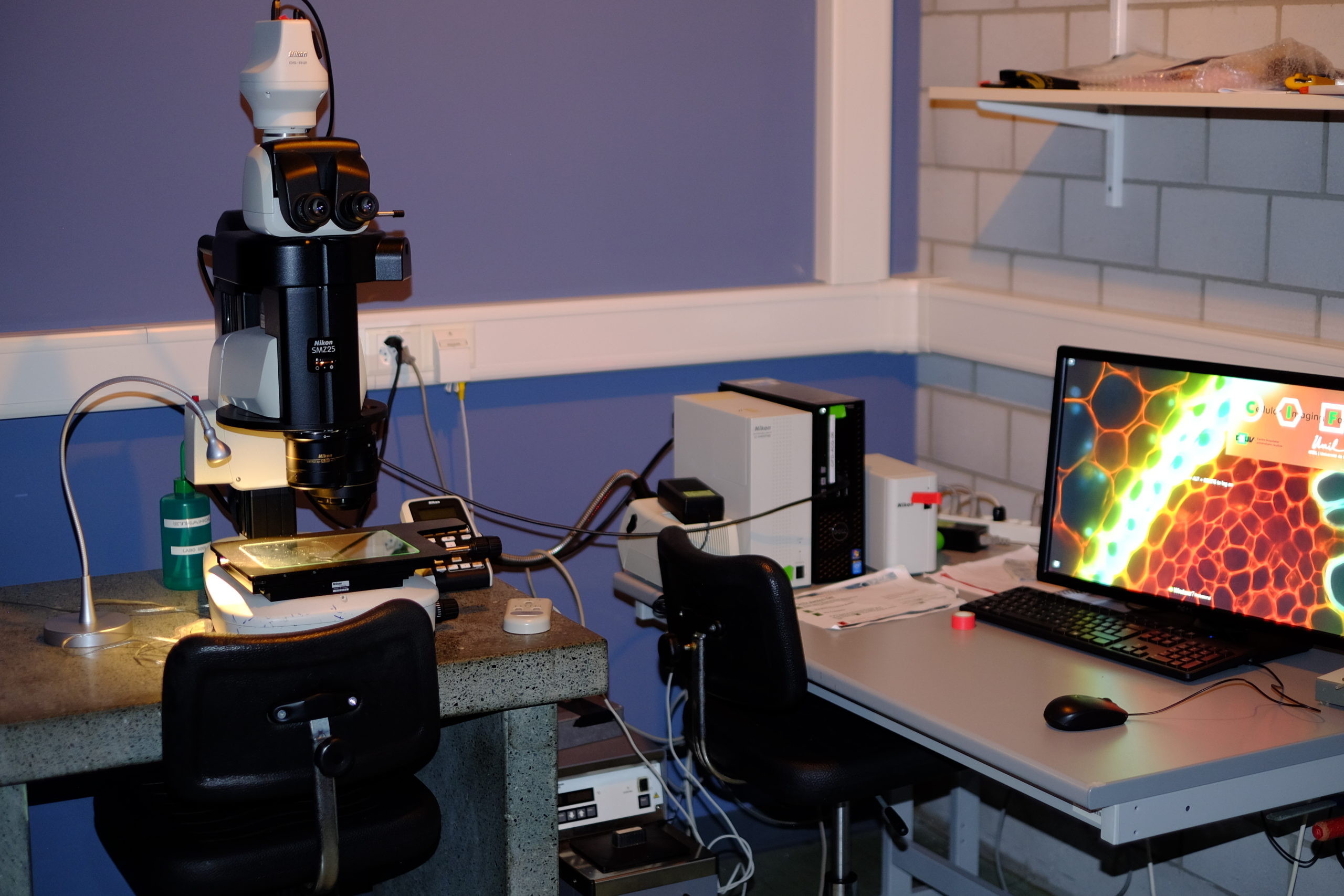
Stereomicroscopy
The stereo, stereoscopic or dissecting microscope is an optical microscope variant designed for low magnification observation of a sample, typically using light reflected from the surface of an object rather than transmitted through it. The instrument uses two separate optical paths with two objectives and eyepieces to provide slightly different viewing angles to the left and right eyes. This arrangement produces a three-dimensional visualization of the sample being examined.[1] Stereomicroscopy overlaps macrophotography for recording and examining solid samples with complex surface topography, where a three-dimensional view is needed for analyzing the detail.
Nikon SMZ25 Stereomicroscope | Macroscope
| Stand |
|
|
Illumination |
|
|
Filters |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Cameras |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Leica Macroscope
Image Processing
[Under construction]