Acquisition systems
Confocal
Confocal microscopy, most frequently confocal laser scanning microscopy (CLSM) or laser confocal scanning microscopy (LCSM), is an optical imaging technique for increasing optical resolution and contrast of a micrograph by means of using a spatial pinhole to block out-of-focus light in image formation.[1] Capturing multiple two-dimensional images at different depths in a sample enables the reconstruction of three-dimensional structures (a process known as optical sectioning) within an object.
Olympus FluoView 3000
|
Illumination |
|
|
Camera |
Olympus DP80 Sensor:CCD color |
|
Lasers |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Detector type |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
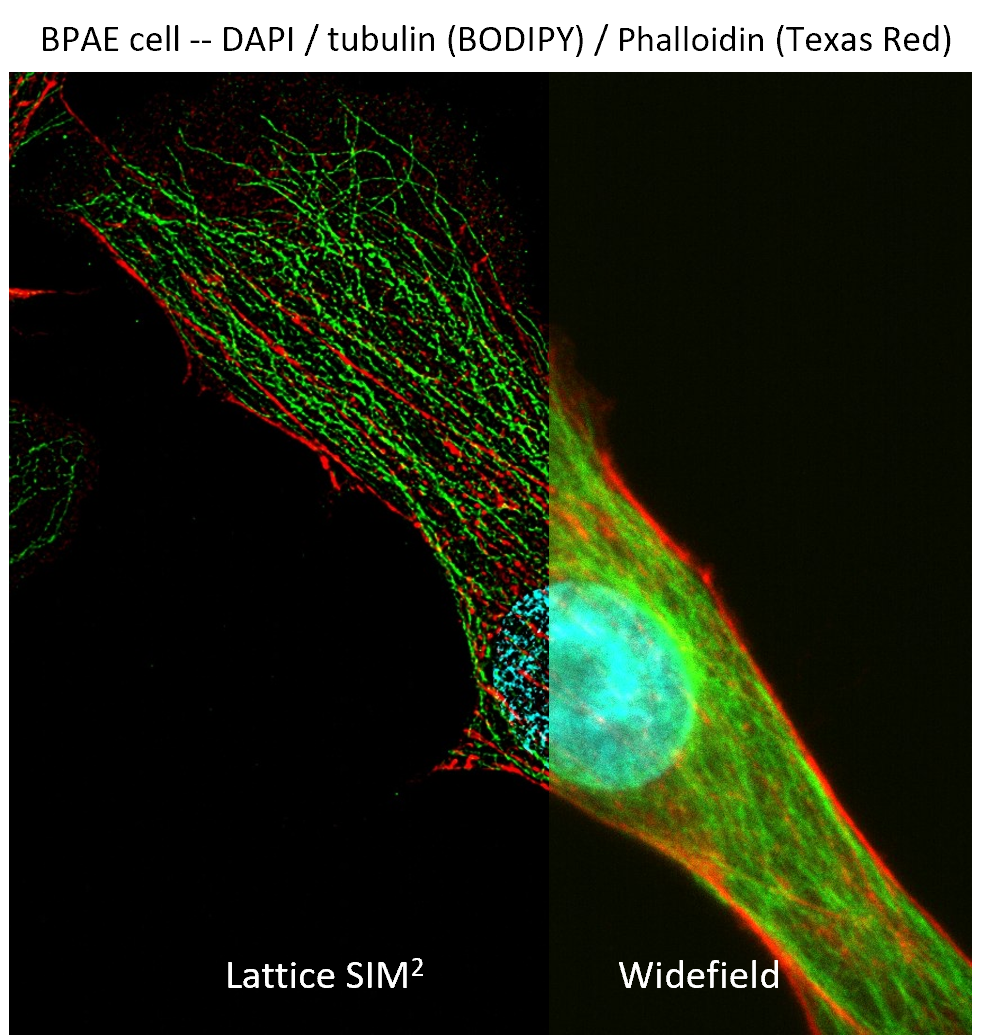
Super Resolution Microscopy
Super resolution microscopy goes beyond the limitations of conventional microscopy by allowing researchers to visualize intricate details at the sub-organelle level. The microscope can incorporate advanced techniques such as Lattice SIM², SIM² Apotome, widefield DIC, SMLM, and TIRF, enabling the correlation of images from the same sample to reveal molecular details with exceptional clarity and precision. This cutting-edge technology provides insights into cellular structures and processes at scales previously unattainable, making it a powerful tool for biological research across various applications.
Zeiss Elyra 7
|
Model |
|
|
Widefield Illumination |
|
|
Lasers |
|
|
Filters |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Incubation |
|
|
Cameras |
|
|
Imaging Modalities |
|
|
Software |
|
|
Room |
|
Spinning Disk | Time-Lapse
Spinning-disk (Nipkow disk) confocal microscopes use a series of moving pinholes on a disc to scan spots of light. Since a series of pinholes scans an area in parallel, each pinhole is allowed to hover over a specific area for a longer amount of time thereby reducing the excitation energy needed to illuminate a sample when compared to laser scanning microscopes. Decreased excitation energy reduces phototoxicity and photobleaching of a sample often making it the preferred system for imaging live cells or organisms.
Nikon Ti2 | Yokogawa CSU-W1
|
Widefield LED Illumination |
Lumencore Spectra X LED
|
|
Filter Cubes |
|
|
Spinning Disk laser excitation |
Omicron modified LightHUB+
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Cameras |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Widefield | Fluorescence
The majority of fluorescence microscopes, especially those used in the life sciences, are of the epifluorescence design shown in the diagram. Light of the excitation wavelength illuminates the specimen through the objective lens. The fluorescence emitted by the specimen is focused to the detector by the same objective that is used for the excitation which for greater resolution will need objective lens with higher numerical aperture. Since most of the excitation light is transmitted through the specimen, only reflected excitatory light reaches the objective together with the emitted light and the epifluorescence method therefore gives a high signal-to-noise ratio. The dichroic beamsplitter acts as a wavelength specific filter, transmitting fluoresced light through to the eyepiece or detector, but reflecting any remaining excitation light back towards the source.
Zeiss | Widefield
| Stage |
|
|
Illumination |
|
|
Filter Cubes |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Cameras |
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Slide scanner
A slide scanner allows you to digitize full microscopy slides in an automated manner. This is a high througput system able to process around a hundred of slides in one batch.
Zeiss Axioscan 7
|
Illumination |
Zeiss Colibri 7 Type R[G/Y]CBV-UV
|
|
Filters |
|
|
Objectives |
|
|
Stage |
|
|
Contrast |
|
|
CO2 | T° control |
|
|
Cameras |
Color camera
Black and White Camera
|
|
Optional modules |
|
|
Software |
|
|
Room |
|
Image Processing
[Under construction]